
How MRMC leverages expertise and relationship-building with industry.
The U.S. Army Medical Research and Materiel Command (MRMC) aims to lead the advancement of military medicine and responsibly create, develop, deliver and sustain medical capabilities for the warfighter. MRMC knows the importance of industry and building relationships, but understands that sometimes industry can’t reach us or doesn’t know how to communicate with us. It’s an important issue to address: Simply put, MRMC can’t get products to the end of cycle without industry.
Six laboratories make up the command’s core science and technology capability; these specific areas of biomedical research are staffed by highly qualified military and civilian scientists and support personnel. There are five additional MRMC subordinate commands that focus on command requirements—such as medical materiel development, logistics and contracting—to complete the life cycle management of medical materiel.
MRMC partners with government agencies, academic institutions and commercial entities to identify solutions that address critical gaps in Military Health System medical research and development (R&D) programs.
MRMC has several tools to assist small businesses and help build relationships. The MRMC “Gateway to Partnerships” guide went live on the MRMC website in November 2017 as a reference to help businesses better understand what we do and how to partner with us.

The NDIA Army Science & Technology Symposium & Showcase focused on the future of warfighting and demonstrated how the future of warfighting is changing with technology modernizations. (Photo by Christina Watson, USAMRMC Public Affairs)
DECISION GATE
MRMC understands that there are many challenges to overcome on both sides when working with small businesses. For example, there may be difficulty attracting and maintaining qualified personnel, staff can get frustrated with current government processes that could use improvement, and some government processes can take longer than expected to complete.
To ensure that the acquisition system runs efficiently, MRMC uses its decision-gate process with every medical R&D product that enters the pipeline. Decision gate provides a governance structure for the development of products throughout the acquisition process, and features event-driven milestones that allow successful products to move along the development pathway. The decision-gate process was implemented in 2005 as a way for MRMC to manage its medical materiel development efforts. It is an overarching process that integrates DOD acquisition processes with the U.S. Food and Drug Administration requirements and industry business practices. The goal is to focus materiel development efforts on meeting DOD requirements as quickly and efficiently as possible.
FORUMS AND EVENTS
Forums and events are great opportunities for MRMC commanders and their staff to learn about other R&D efforts and their importance within the command. During forums and events, leaders and industry can have face-to-face discussions, network and gain knowledge through professional development. Such events allow government, academic and private sector experts to explore emerging medical technologies and their potential roles in supporting military medicine. For example, the Telemedicine and Advanced Technology Research Center (TATRC) 4th Annual Open House and Technology Demonstration in September 2018 educated the military medicine community and external partners about TATRC’s focus on supporting military readiness through innovative technologies across the Military Health System.
VENDOR DAYS
Military Health System Vendor Days are cooperative ventures by the military services, located at Fort Detrick, Maryland. Typically held seven times a year, the event focuses on assisting the services’ medical agencies with strategic market analysis of products and technologies that may be applicable to austere medical environments.
The agencies involved are:
- Defense Health Agency Medical Logistics Division.
- U.S. Army Medical Research and Materiel Command.
- U.S. Army Medical Materiel Agency.
- Naval Medical Logistics Command.
- U.S. Air Force Medical Operations Agency.
- U.S. Marine Corps Systems Command.
RESEARCH SYMPOSIUMS
Like open houses, conferences and symposiums facilitate networking, collaboration with peers and small-group discussion sessions. One such event, the Military Health System Research Symposium, held in Kissimmee, Florida, Aug. 20-23, is considered by many as a premier DOD medical research meeting, bringing together military and civilians to collaborate and create partnerships.
MRMC also participated in the 2018 National Defense Industrial Association’s Army Science & Technology Symposium & Showcase, held Aug. 21-23 at the Walter E. Washington Convention Center in Washington. Events such as this allow representatives and leadership to engage with industry and discuss ongoing research projects and capabilities.

The 2018 Military Health System Research Symposium focuses on the Warfighter’s unique medical needs. The Symposium consisted of more than 75 break-out sessions, 90 exhibits and 1,400 poster presentations. (Photo by Leticia Hopkins, USAMRMC Public Affairs)
MECHANISMS FOR COLLABORATION
Two congressionally mandated programs—Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR)—were created to promote technological innovation and economic growth among small businesses through federal investment. SBIR and STTR allow small, high-tech U.S. businesses and academia the opportunity to provide innovative research and development solutions in response to critical DOD needs.
Five federal agencies participate in both SBIR and STTR:
- DOD
- NASA
- Department of Health and Human Services.
- National Science Foundation.
- Department of Energy.
Six federal agencies participate in the SBIR program only:
- Department of Agriculture.
- Environmental Protection Agency.
- Department of Education.
- Department of Homeland Security.
- Department of Transportation.
- Department of Commerce.
CRADAs
The preferred vehicle for technology transfer is a cooperative research and development agreement (CRADA), which allows MRMC investigators to collaborate with scientists in industry and academia and work toward a common research goal. CRADAs allow federal laboratories to collaborate on R&D with nonfederal parties and are used for nondisclosure agreements, material transfer agreements and complex R&D collaborations. Visit the MRMC’s Office of Research and Technology Applications (ORTA) at http://technologytransfer.amedd.army.mil/index.cfm to learn more about the possibility of creating a CRADA with MRMC.
MRMC also has technologies available for licensing. For licensing opportunities, please contact ORTA at USArmy.Detrick.MEDCOM-MRMC.List.ORTA@mail.mil.
OTHER WAYS OF DOING BUSINESS
The Medical Technology Enterprise Consortium (MTEC) consists of industry, academia and other entities organized and operated through a nonprofit corporation. MTEC currently has more than 200 members and is seeking new members from the following areas:
- Small and large businesses.
- Academic medical research organizations.
- Not-for-profit organizations.
- Nontraditional government contractors.
MRMC works with MTEC through a prototype other transaction agreement, which provides a flexible method to combine public and private resources to focus research, prototype development and commercialization on specific shared military and civilian medical technology needs.
An other transaction agreement is a funding vehicle used by federal agencies for obtaining or advancing R&D or prototypes. It’s generally used with small businesses and nontraditional contractors, but traditional contractors can be funded through that way if certain criteria are met. Other transaction authority is not a contract or assistance agreement.
The other transaction agreement mechanism enables discussions with potential performers throughout the process to develop better requirements for projects to meet the needs of both military and commercial health care markets. This type of interaction is usually not allowed through typical contracting and grants processes.
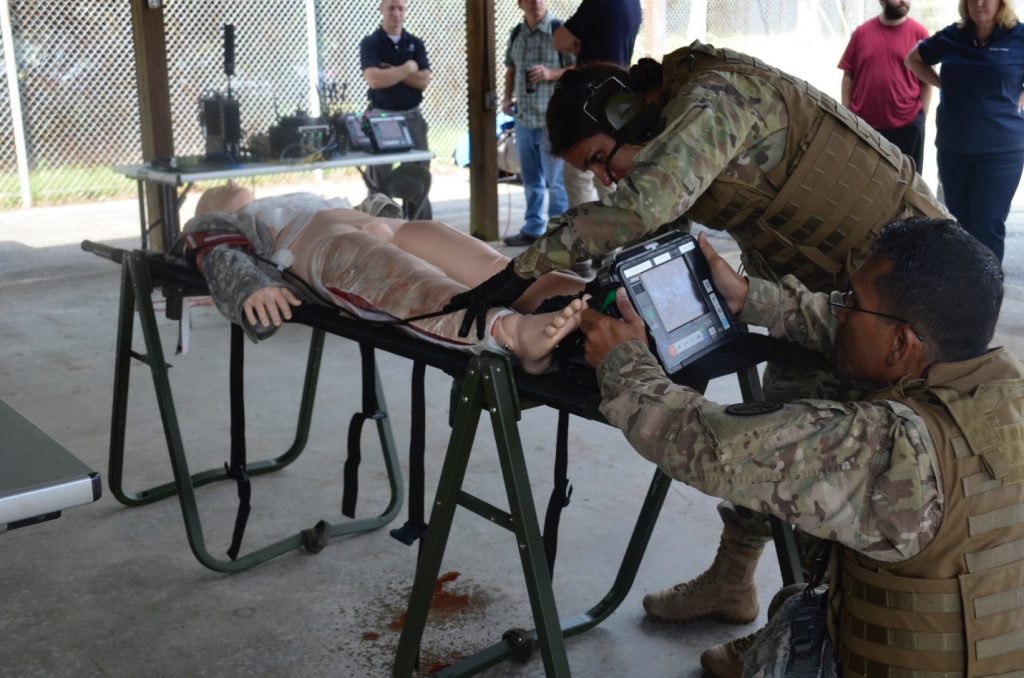
Field medics perform a simulated escharotomy using the Remote Diagnostic Technologies i2i Solution (Tempus Pro) at TATRC, Fort Detrick, Sept. 7. (Photo by Elizabeth Lamie, USAMRMC Public Affairs)
MAXIMUM OPPORTUNITIES
MRMC is committed to ensuring that businesses are provided with maximum opportunities to compete for procurements. There are several systems and portals that enable academia and industry to submit ideas and research.
The command recognizes that unsolicited proposals with unique and innovative products or ideas that have been developed outside of the government can help it accomplish its mission. MRMC uses the New Products and Ideas (NPI) system as a mechanism to evaluate new products and ideas, currently configured or in development, that support the mission.
The NPI is a web-based system that provides a means for our subject matter experts to assess these products and ideas, evaluate their applicability and provide feedback to the submitter. It gives academia and industry an opportunity to showcase their products or ideas without needing to travel to Fort Detrick and without giving anyone an unfair competitive advantage. After the submitter enters data, an expert receives the information and provides feedback within 60 days.
Additionally, NPI gives the public direct access to the scientific expertise of DOD. It is open to the public so anyone can submit ideas or information. NPI has been a successful avenue for submission, giving submitters the opportunity to obtain feedback without an extensive process.
No funding is associated with NPI, but it allows for constructive feedback that can help with developing and refining the ideas that are submitted. Recent submissions to NPI resulted in further collaborative ventures for products in such areas as combat casualty care, military operational medicine, burn treatment, infectious diseases and surgery.
OFFICE OF SMALL BUSINESS PROGRAMS
The mission of MRMC’s Office of Small Business Programs is to maximize opportunities for various categories of small businesses to compete for procurements as either a prime or subcontractor and to forge strategic business alliances. The office is committed to supporting small businesses in their pursuit to provide products, services and solutions that sustain our nation’s warfighters. The organization’s goal is to ensure that small businesses remain an integral part of MRMC’s business solutions. For information about the MRMC Small Business Office, go to http://smallbusopps.amedd.army.mil/.
CONCLUSION
Military forces will continue to seek advanced medical products and devices to support their missions. The process needs streamlining—with requirement approval currently taking two to three years and technology moving so quickly, the technology may become obsolete before it is even implemented. The goal is to get products requested by the warfighters in their hands as quickly as possible.
Although the acquisition process may provide challenges, it also includes a necessary system of checks and balances to ensure the delivery of safe, effective, affordable and sustainable solutions to service members.
For more information and for details on working with MRMC, go to http://mrmc.amedd.army.mil.
ELIZABETH LAMIE is a writer and content development specialist for the Public Affairs Office at Fort Detrick, providing contract support to MRMC for eLittle Communications Group. She has more than 15 years of experience researching and writing for publications and companies, and holds a B.A. in English from George Mason University.
This article will be published in the January – March 2019 issue of Army AL&T magazine.
Subscribe to Army AL&T News – the premier online news source for the Army Acquisition Workforce.
![]() Subscribe
Subscribe

