
By Col. Matthew G. Clark and Hannah Feldman
Disclaimer: The views expressed in this article reflect the views of the authors and do not purport to reflect the official views of the Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense, the U.S. Army, the Department of Defense or the U.S. government.
The Department of Defense (DOD) acquisition process is lengthy and arduous, especially for highly technical products, including medical countermeasures (MCMs) like drugs, devices, vaccines, and diagnostic equipment. In the medical space, add to this process a test and evaluation organization like the U.S. Food and Drug Administration which must approve products prior to fielding. Consequently, it can take upwards of 15-20 years and more than $2.6 billion to develop and field a new drug or vaccine.
In the face of an imminent threat, we don’t have the luxury of spending more than a decade sequentially testing, developing, and fielding a medical countermeasure. Meeting the needs of rapid acquisition requires new approaches for product development. At least for medical products and systems requiring exceptionally high reliability, a new authority like Middle Tier Acquisition, flexible thinking, and Overlapping, Iterative, and Incremental Development provide ways to accelerate product development.
Opioids and the Need for Acquisition Speed
Today, one such threat requiring rapid development comes in the form of opioids, and a solution is needed now. Declared a public health emergency in 2017, our Nation is in the midst of an unprecedented epidemic with prevention, treatment for addiction, and overdose reversal drugs critical for fighting back against this threat. The crisis also presents a threat to the military. Pharmaceutical-Based Agents, or PBAs, are compounds derived from pharmaceuticals with the intent to incapacitate or kill. Some PBAs, such as fentanyl and its ultra-potent derivatives, are readily available and lethal at incredibly small doses; landing them on the 2017 Chemical Terrorism Risk Assessment list. There is a critical need to deliver a medical countermeasure that can reverse the effects of accidental or intentional opioid exposure for individuals like first responders and military personnel who support civilian law enforcement.
Opioids do not discriminate. U.S. troops and first responders are equally vulnerable to PBA exposure. At the Joint Project Management Office for Medical Countermeasure Systems (MCS)—under the Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear (CBRN) Defense—we are exploring new ways to accelerate the acquisition process to get MCMs into the hands of Joint, Interagency, Intergovernmental, and Multinational, or JIIM, users faster. MCS is a component of the DOD’s Chemical Biological Defense Program and is charged with developing vaccines, diagnostics, devices, and therapeutic drugs that protect, quickly diagnose, and treat troops exposed to CBRN threats.
Even though there are opioid MCMs available for commercial use, due to various factors, they do not meet the operational requirements for Service Members to complete their mission in a chemical warfare setting. These MCMs require further development both in terms of dose and the method for administration in an austere or mass casualty environment.
MCS is leveraging the FDA-approved drug naloxone, which treats the effects of opioid poisoning. While naloxone is considered the “gold standard” for treating an opioid overdose, the drug is only approved for use in low doses and concentrations; for the military and first responders, a much higher dose and new formulation is required for treatment that supports readiness and mission success. MCS is leveraging the drug’s existing FDA approval to seek new approvals to increase the dose and concentration, and place it into an autoinjector device that allows rapid treatment on the battlefield or in other scenarios requiring rapid countermeasure administration.
Middle Tier Acquisition
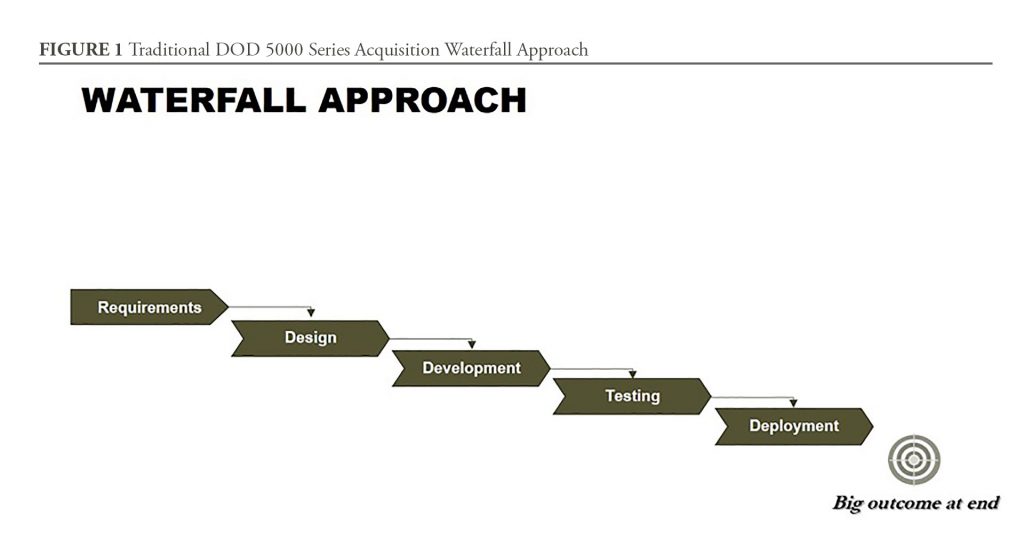
To quickly meet DOD needs, the Chemical Defense Pharmaceuticals product office at MCS is also leveraging partnerships, rapid prototyping, and Middle Tier Acquisition to quickly develop and field the naloxone autoinjector for JIIM users. Middle Tier Acquisition was authorized in the FY2016 National Defense Authorization Act, and is an attempt to get capabilities into the field in less than five years from requirements approval. This approach streamlines bureaucratic elements such as the requirements process, testing, deployment, and adaptation of prototypes using proven technology. As discussed below, a new capability can be developed faster using overlapping iterations and increments, and through adapting, combining, and updating existing solutions. Under this new authority, designated programs are not subject to the traditional acquisition waterfall framework outlined in the DOD Directive 5000.01 (See Figure 1). Essentially, both the DOD 5000 series and this new authority provide product managers all the flexibility they need to streamline acquisition and deliver capabilities fast.
For example, there are currently no suitable commercially available naloxone autoinjectors that meet military requirements. Utilizing the rapid prototyping approach of Middle Tier will allow the 10 mg naloxone autoinjector to complete prototype development well within five years, significantly decreasing the time for the drug-device product to receive FDA approval and be fielded to U.S. troops. This approach builds on existing or innovative technologies to develop prototypes of new capabilities that meet emerging military needs.
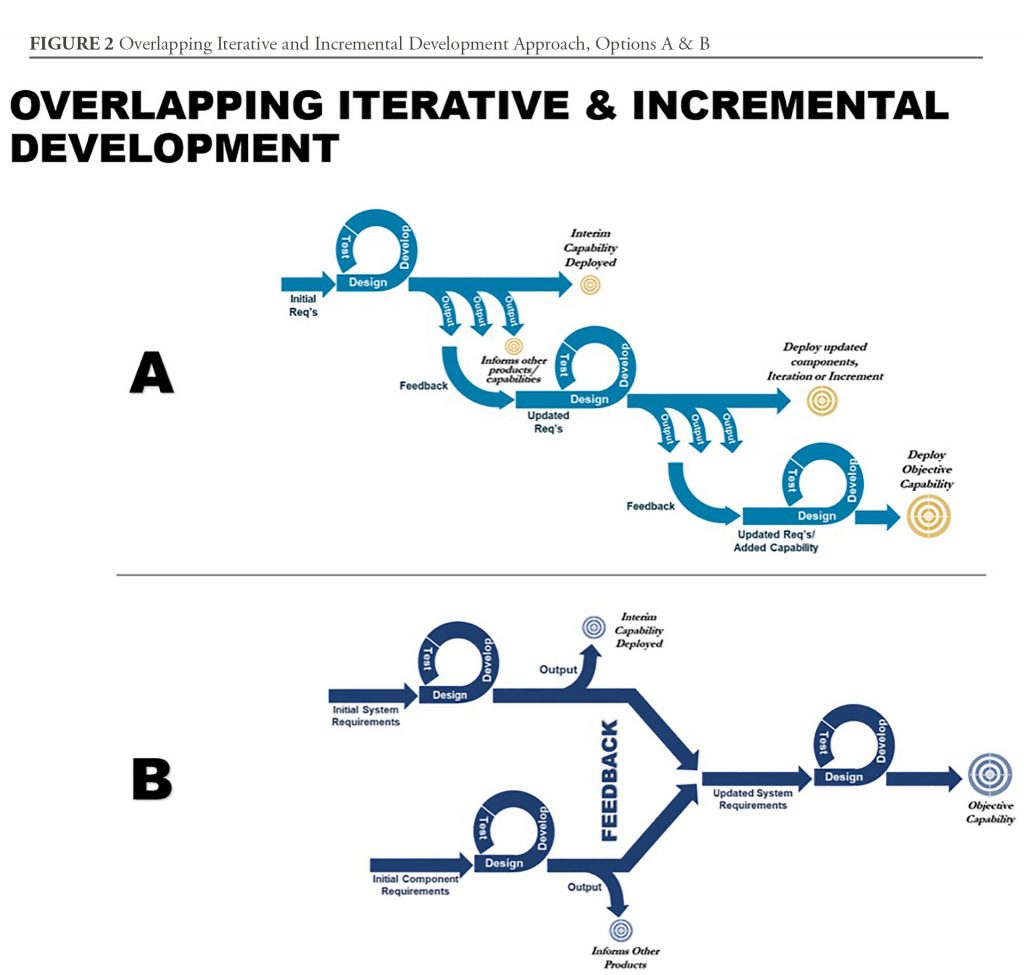
Streamlining through Overlapping, Iterative, and Incremental Development
One of the main challenges, however, is to adapt acquisition planning and activities created by teammates who were trained to use traditional acquisition thinking. Simply, the waterfall approach of the Defense Acquisition System—while great for teaching the elements of product development—is insufficient for streamlining a more rapid acquisition. There is a need to explore and teach acquisition professionals new methods for rapid product development. Interestingly, the DOD 5000 series provides such flexibility, but, historically, acquisition professionals were expected to adhere closely to, and never deviate from, the prescribed process.
In the software space, “Agile” development encourages delivery of low fidelity capabilities as a step-wise process moving toward an objective capability; however, in tightly engineered systems with exceptionally high reliability requirements, that approach will not work. Medical test and evaluation requirements and approval timelines do not allow for short sprints, especially in a way that produces interim capabilities that at least partially meet the needs of JIIM users. However, at MCS, we are finding that teaching teams to use an Overlapping, Iterative, and Incremental Development model (See options in Figure 2) is better at providing an interim and initial medical capability sooner, and has the potential to move the system quickly towards its objective capability. This approach requires an open mind and a willingness to think flexibly about how to build a process that meets requirements.
Therefore, this approach involves simplifying product development down to the most basic components, preferably with a focus on components that can either be delivered as interim capabilities or those involving the most challenging or complex development. Once the deliverable and complex components or iterations are understood, the product team can further determine how the product can be simplified to ensure rapid acquisition. This activity may also reveal trade space to streamline opportunities for interim fielding.
Overlapping activities accelerate development by allowing work to be done on multiple elements simultaneously. Combining this with an iterative and incremental approach provides the risk mitigation and accelerated delivery of medical product development. This, too, is important, because risk management, while it can divert the attention of product managers, can also provide information that can inform decisions for acceleration or termination of unsuccessful efforts sooner. Research on this topic reveals that those who make decisions earlier are better able to convert investment into a stronger return.
Important Considerations
Arraying activities over time is a key activity for success when using overlapping iterations and increments. Streamlining cannot occur without attention to this part of the process. Yet, this does not mean teams will do less before they act. Indeed, teams have to invest more in information gathering before acting. Arraying activities appropriately requires as much information as is reasonably possible through requests for information and other interactions with potential performers (e.g., through an Other Transaction Authority consortium, if one is available). This process allows products that are high risk to inform knowledge points, guide engineering decisions affecting components, and link related developments.
Lastly, an Overlapping, Iterative, and Incremental approach also means the program office will likely spend a large amount of time on stakeholder engagement to address questions and concerns from the acquisition community about using this approach. However, if the team continually listens to and engages the community, and relies on constructive criticism, any potential communication challenges can be easily overcome.
 Attacking the Opioid Medical Countermeasures
Attacking the Opioid Medical Countermeasures
To develop a MCM against opioids, the MCS team used available information from subject matter experts to make early decisions that were risk-informed. Stakeholders used a broad-based teaming approach and created teams that could both collaborate and challenge the assumptions of the product management team. Then, we continually communicated our plans to potential stakeholders (including international partners) and further exploited unexpected opportunities. Eventually, we were able to develop clarity around what was achievable using Middle Tier Acquisition. Using this approach, we expect to deliver a capability against this important threat much faster than the traditional 12-15 year average for standard drug development.
“With the opioid crisis in the U.S., and the recent nerve agent attacks across the world, we need to make sure our troops are protected from opioid exposure,” said Col. David P. Hammer, Joint Project Manager for MCS. “This acquisition approach allows us to provide a critical medical countermeasure to the Warfighter quicker than we could otherwise.”
With this authority and approach, the 10 mg naloxone autoinjector should receive FDA approval in FY2022, and be rapidly fielded in FY2023, significantly lowering the cost and decreasing the schedule to provide this critical capability to JIIM users. We believe that by using new and existing authorities, along with streamlining through Overlapping, Iterative, and Incremental Development, we can change the way we think, teach, streamline, and accelerate Defense acquisition.
__________________________________
Col. Matthew G. Clark, PhD, PMP, is the Joint Product Manager for Chemical Defense Pharmaceuticals within Medical Countermeasure Systems at Fort Detrick, MD. The organization is in the Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense. COL Clark is responsible for managing centralized research, development, acquisition, and integration of FDA-approved medical countermeasures against chemical, radiological, and nuclear threats. The products they develop are used by Joint, Interagency, and Multinational organizations. COL Clark has a Ph.D. in Behavioral Neuroscience from Rutgers University and a B.A. in Psychology from Coe College. He has extensive rapid and deliberate capability development experience in DOD Labs, Iraq, G-3/5/7 of the Army Staff, and USAMRMC and service at the U.S. Military Academy and the U.S. House of Representatives. He also has published on leader development and a host of military relevant topics, while also serving in nonprofit organizations serving the military and veteran community.
Hannah Feldman currently supports the MCS Communications team as a Public Affairs Specialist as an employee of Patricio Enterprises, Inc. She holds a B.A. and M.A. in International Affairs from American University in Washington, DC. Hannah specializes in strategic communications, outreach, and stakeholder engagement.
 This article is a winner in the 2018 Maj. Gen. Harold J. “Harry” Green Awards for Acquisition Writing competition. A special supplement featuring the winning entries is online now, and will accompany the print version of the April – June 2019 issue of Army AL&T magazine.
This article is a winner in the 2018 Maj. Gen. Harold J. “Harry” Green Awards for Acquisition Writing competition. A special supplement featuring the winning entries is online now, and will accompany the print version of the April – June 2019 issue of Army AL&T magazine.
Subscribe to Army AL&T News – the premier online news source for the Army Acquisition Workforce.
![]() Subscribe
Subscribe







